Study design
Observational Study Designs: Case control study
![]() Case-control studies can be useful when a rare event is studied, the sample available is small, or when initial evaluation (pilot study) of an exposure of interest is necessary. In these studies, the study sample is selected based on whether the outcome occurred already.
Case-control studies can be useful when a rare event is studied, the sample available is small, or when initial evaluation (pilot study) of an exposure of interest is necessary. In these studies, the study sample is selected based on whether the outcome occurred already.
Subjects with the outcome of interest are referred to as the cases. Controls are subjects without the outcome. Controls must be found based on pre-specified matching criteria. After finding cases and controls, whether or not they had the exposure of interest is determined. Finding appropriately matched controls can be difficult and selection bias must be considered when using this study design.
In this study design, the ascertainment of exposure is done after the outcome. This means results lack the element of temporality and therefore causality cannot be implied from these studies. Case control studies do not answer whether an exposure is associated with an outcome. These studies can only determine whether a subject with the outcome of interest was more/less likely to have the exposure of interest compared to the controls, which makes the level of evidence from this study designs lower than cohort studies.
Figure 1. Case-control Design
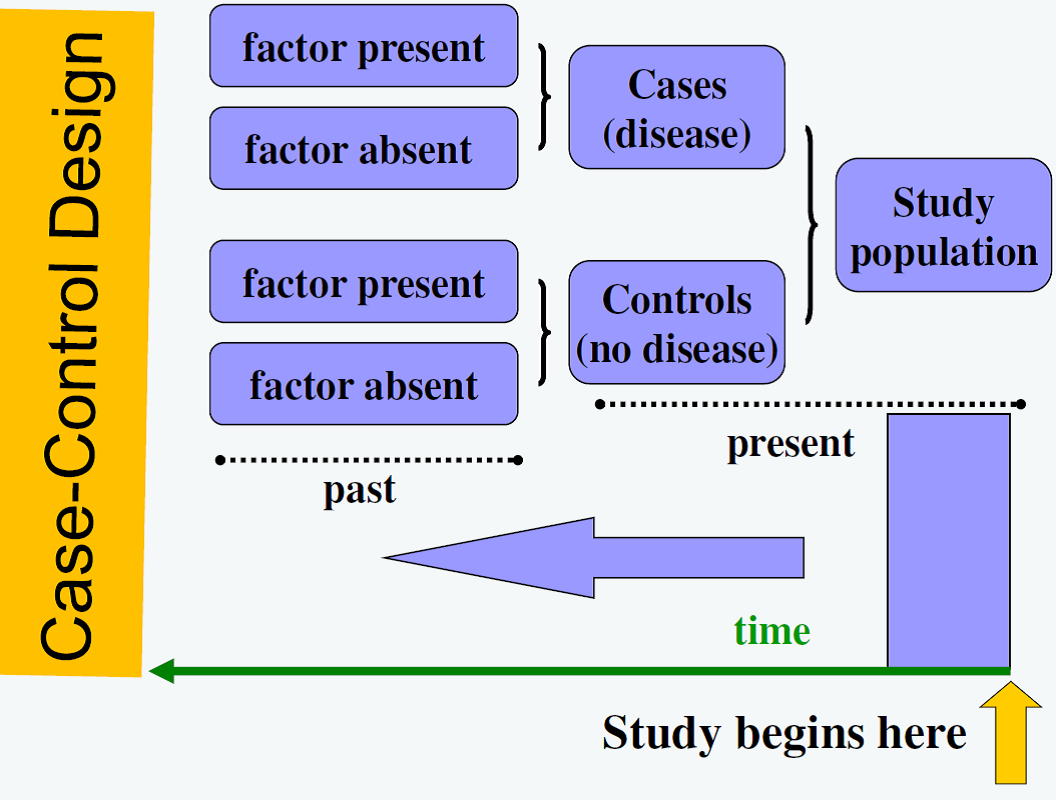
Figure from: Niyonsenga, T. Foundations of Public Health Epidemiology (HLTH 5188). School of Population Health. University of South Australia.
Advantages
- Good to investigate rare outcomes
- Good to investigate outcomes with long periods between the exposure and outcome development
- Can be less costly
Disadvantages
- Not good for rare exposures
- They are subject to a lot of bias and confounding
- Difficulties in identifying appropriate controls
- Cannot infer causality because of the lack of temporal association
- Cannot calculate incidence of disease