Study design
Epidemiological Studies Overview
![]() Epidemiological studies can be descriptive and/or analytical. Descriptive studies are used to describe exposure and disease in a population, and can be used to generate hypotheses, but they are not designed to test hypotheses. Analytical studies are designed to evaluate the association between an exposure and a disease or other health outcome, and therefore are designed to test hypotheses. This module will focus on analytical epidemiological studies.
Epidemiological studies can be descriptive and/or analytical. Descriptive studies are used to describe exposure and disease in a population, and can be used to generate hypotheses, but they are not designed to test hypotheses. Analytical studies are designed to evaluate the association between an exposure and a disease or other health outcome, and therefore are designed to test hypotheses. This module will focus on analytical epidemiological studies.
Epidemiological studies can also be prospective or retrospective. A prospective study is one where the study starts before the exposure and outcome are ascertained. A retrospective study is one where the study starts after the exposure (and probably outcome) has been ascertained.
Other terminology important for epidemiological studies design module:
- Exposure: the risk factor (agent, experience, procedure, etc) that is suspected to have caused the disease. In statistical terms, this is your independent variable.
- Outcome: the disease, endpoint that you are measuring. In statistical terms, this is your dependent variable.
There are several types of epidemiological studies (Figure 1). These fall under experimental and observational designs. The most commonly used analytical study designs are listed here and are covered in this module.
A. Experimental
(i) Randomized clinical trials
(ii) Other non-randomized interventional studies
B. Observational (non-experimental)
(i) Cross-sectional study
(ii) Case control study
(iii) Cohort study
(iv) Ecological study
Figure 1. Types of Epidemiological Studies
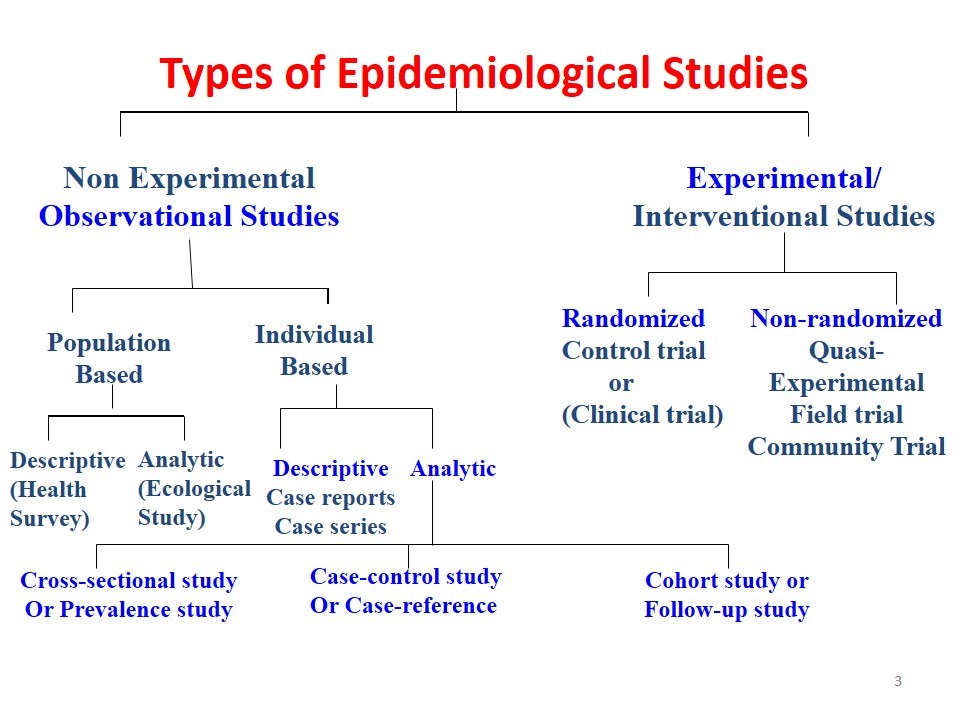
Figure from: http://howmed.net/community-medicine/study-designs/
References:
- Rothman, K. J., Greenland, S., & Lash, T. L. (2008). Modern Epidemiology, 3rd Edition. Philadelphia, PA: Lippincott, Williams & Wilkins
- Gordis, L. (2009). Epidemiology. Philadelphia: Elsevier/Saunders.
NMHRC Dimensions of Evidence
Individual studies included in a systematic literature review should be assessed using the NHMRC dimensions of evidence and provides levels of evidence appropriate for the most common types of research questions. Further information can be found here.