Study design
Observational Study Designs: Introduction
![]() Observational studies are studies where the exposure you are evaluating is not assigned by the researcher. This means that no randomization occurs as part of the study and therefore the selection of subjects into the study and analysis of study data must be conducted in a way that enhances the validity of the study.
Observational studies are studies where the exposure you are evaluating is not assigned by the researcher. This means that no randomization occurs as part of the study and therefore the selection of subjects into the study and analysis of study data must be conducted in a way that enhances the validity of the study.
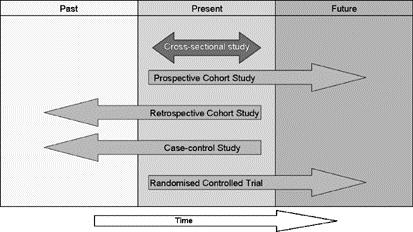
Observational studies can be prospective, retrospective, or cross-sectional. When the exposure was determined can be used to define whether the study is prospective or retrospective. If the exposure was recorded before the outcome occurs the study is typically deemed a prospective study. If the exposure was recorded after the outcome has occurred then the study is typically deemed a retrospective study. A cross-sectional study is one where exposure and outcome are determined at the same time.
The three most common types of observational studies are cross-sectional, cohort, and case-control, which are covered in this document. For other variations and mixed study design please refer to:
- Rothman, K. J., Greenland, S., & Lash, T. L. (2008). Modern Epidemiology, 3rd Edition. Philadelphia, PA: Lippincott, Williams & Wilkins
- Gordis, L. (2009). Epidemiology. Philadelphia: Elsevier/Saunders.
Figure 1. Direction of temporal observation by study design1
Tip:
Become familiar with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement: http://www.strobe-statement.org/. This is a collaborative initiative of researchers and journal editors involved in the conduct and dissemination of observational studies.1
References:
1.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine 2007;147:573-7.
2. Figure from: Levin KA. Study design III: Cross-sectional studies. Evidence-based dentistry 2006;7:24-5.