Experimental Study Designs: Randomised clinical trials
Considered the “gold standard” of research designs because provides the most convincing evidence of the relationship between exposure and outcome. Only randomized control trials can fulfill the criteria for causal inference, which means to establish a cause and effect relationship.
One commonly used set of causal inference criteria was proposed by Bradford-Hill.
Bradford-Hill Criteria for Casual Inference1
- Temporal relationship
- Strength of the association
- Biologic plausibility
- Dose–response relationship
- Replication of the findings
- Effect of removing the exposure
- Alternate explanations considered
- Specificity of the association
- Consistency with other knowledge
In a randomized control trial, subjects are enrolled into the study based on a pre-specified set of inclusion and exclusion criteria. Then subjects are randomized into into one treatment (i.e. treatment group) or a different treatment or placebo (i.e. comparison or control group). This treatment, or exposure, is what you mean to evaluate as the “cause” for a specific outcome. Randomization is hallmark of these experiments and the reason why they are considered the gold standard of scientific evidence. The goal of randomization is to eliminate differences between the groups, or create groups that are as similar as possible. By doing this you remove any bias or confounding that different characteristics of the groups could have on the specific exposure-outcome relationship you are evaluating. After randomization, subjects are followed for a period of time and the incidence of the event that you are interested is measured and compared.
Advantages:
- Gold standard of research designs
- Provides convincing evidence of causal relationship
- High internal validity
Disadvantages:
- Very expensive
- Limited external validity
- Not appropriate for certain questions. Some maybe unethical, cannot be practically carried out, or if rare events are being studied.
- For example, a randomized control trial for the effectiveness of parachutes cannot be conducted. See http://www.ncbi.nlm.nih.gov/pmc/articles/PMC300808/2
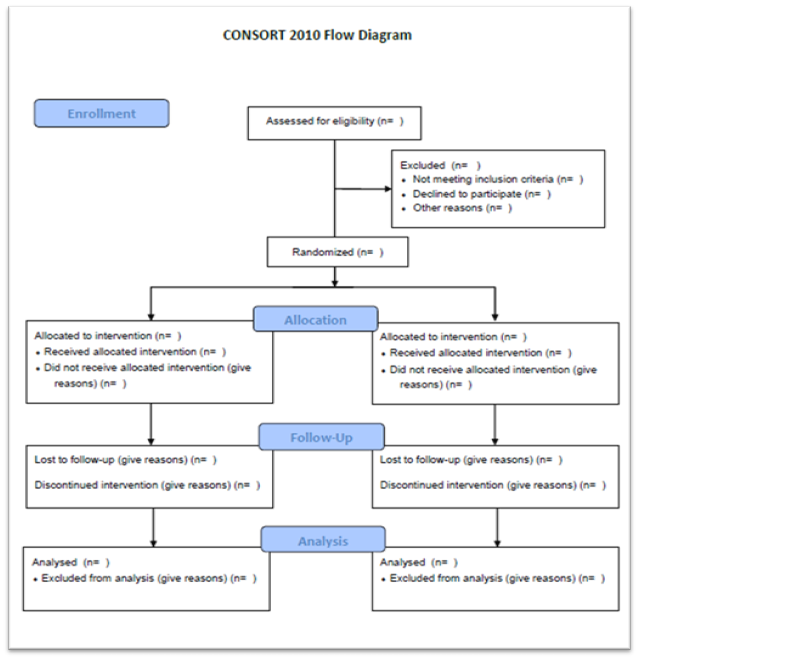
Tip: Become familiar with the CONsolidated Standards of Reporting Trials (CONSORT) 2010 guidelines. Figure 1 is the flowchart proposed by the CONSORT 2010 guideline. This flowchart shows the recommended design and structure of a clinical trial. Becoming familiar with this publication guideline is essential for those interested in designing and conducting this type of study.3
Figure 1. CONsolidated Standards of Reporting Trials (CONSORT) 2010 guideline Flowchart3
Figure from: http://www.consort-statement.org/consort-statement/flow-diagram
References:
- Austin Bradford Hill, “The Environment and Disease: Association or Causation?,”Proceedings of the Royal Society of Medicine, 58 (1965), 295-300.
- Smith , G. C. S. , & Pell , J. P. ( 2003 ). Parachute use to prevent death and major trauma related to gravitational challenge: Systematic review of randomized controlled trials . British Medical Journal , 327 , 1459 – 1461 .
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med 2010;152.