Minimal Inhibitory Concentration (MIC)
When resistance to a certain antimicrobial arises, it is often useful to determine the minimal inhibitory concentration (MIC) of that drug for that organism so that dosages can be adjusted to prevent treatment failure. The MIC of a drug is the lowest concentration capable of completely inhibiting the growth of a test organism.
There are a number of methods suitable for determining the MIC of antimicrobials. Choice of method is determined by the type of organism, fastidious organisms are generally tested using an agar incorporation technique. For less fastidious organisms, a broth dilution technique in Mueller Hinton broth is suitable. In this test, a log phase culture is diluted to the point where it is no longer visually turbid and then mixed with a series of antibiotic dilutions. End point is determined by observing for growth in the wells/tubes after incubation. Broth dilution is particularly well adapted to automation, where growth in the wells can be monitored with a spectrophotometer.
In addition, a commercial system which utilises an antimicrobial concentration gradient on a strip (E-test) is also available. In this test the strip is placed on a lawn of bacteria on a Mueller Hinton plate, much like a disc diffusion test.
Broth Dilution
Preparation of Inoculum
- Dilute a log phase culture of the test organism in sterile saline to the equivalent of the 0.5 MacFarland turbidity standard and then further dilute the saline suspension in Mueller Hinton broth (MHB) by 1 in 100. You will need 50 μl per well plus a growth control well for the test.
Preparation of Antibiotic Dilutions
- Prepare a solution of antibiotic in Mueller Hinton broth (MHB) which is twice the highest concentration to be tested.
- Add the 100μl of this solution to the first well in the microtitre tray.
- Add 50μl of MHB to the next wells in the row - number of wells depends on how many dilutions need to be tested..
- Remove 50 μl of antibiotic solution from the first well and add it to the second well. Mix with the pipette.
- Using a clean tip, remove 50 μl of solution from the second well and add it to the third well, mix and repeat for the following wells. Discard the excess 50 μl from the last well leaving 50 μl of MHB in the last well as the growth control.
MIC Test
- Add 50 μl of diluted culture to each of the antibiotic wells (this constitutes a 1 in 2 dilution, hence the need for the initial concentration to be twice that required.).
- Incubate at 37oC overnight.
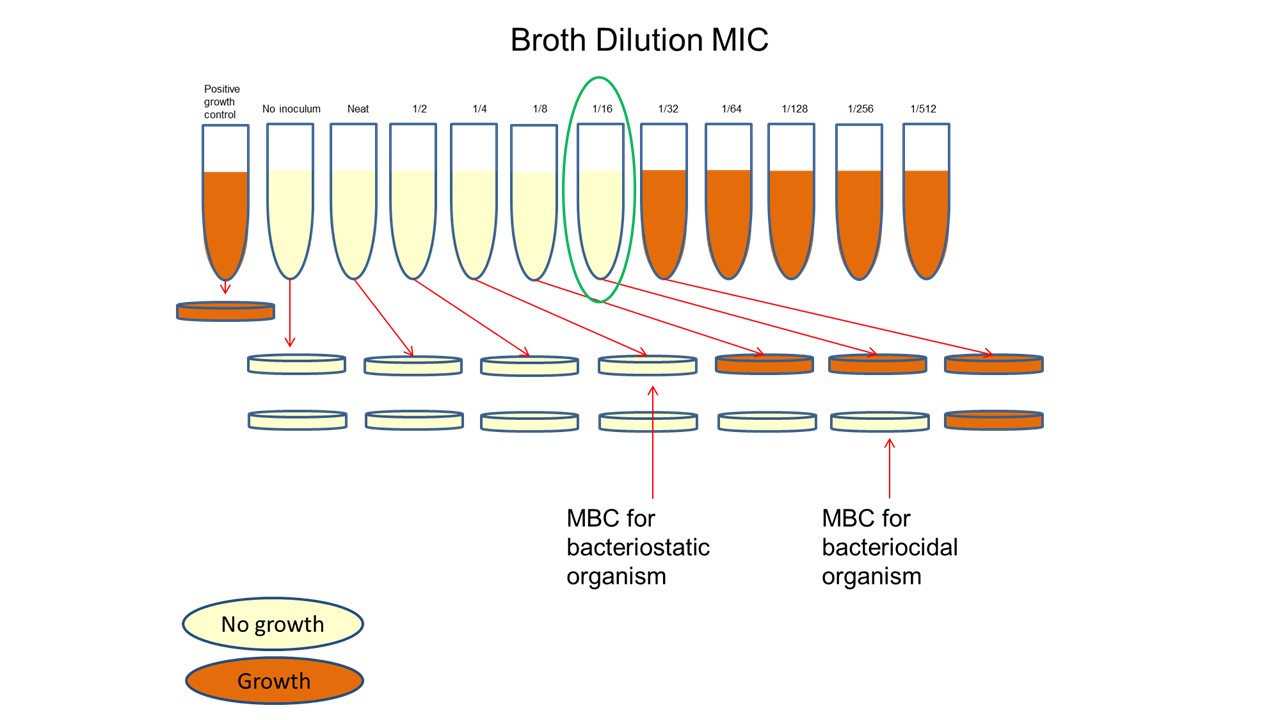
- Record growth by observing turbidity in the wells. Do not shake the trays, and observe a button of bacteria in the base of the wells which have grown. The button is due to the bacteria settling out on the bottom of the wells. Wells which did not grow do not have this button of cells. MIC is the lowest dilution showing complete inhibition of growth. Ensure that the growth control well with no antibiotic has grown.
E-Test
The E-test is a commercial system which is similar in principle to disc diffusion except that the antibiotics are applied to a filter paper strip with a concentration gradient along the strip which is calbrated to certain concentrations. the strip is applied to a lawn of bacteria on a Mueller Hinton agar plate, incubated and the MIC read from the strip where the zone of inhibition intersects it.
Procedure:
- Dilute a log phase culture of the test organism in sterile saline to the equivalent of the 0.5 MacFarland turbidity standard.
- Make a lawn of the organism on a plate using a swab, allow the plates to dry (with the lid on) on the bench for 5 minutes and carefully place the appropriate E-test strip down the centre of the plate.
- Incubate at 37oC overnight in the aerobic incubator.
- Record the result for the E-test by determining where the elliptical zone of inhibition intersects the strip. Units are in μg/ml. Be sure to examine the edges of the zone carefully.
Minimal Bactericidal Concentration (MBC)
Some antibiotics are bacteriocidal, meaning they kill bacteria outright, others are bacteriostatic, meaning they inhibit the growth of bacteria but do not necessarily kill them. For bacteriocidal antibiotics the MIC will be similar to the minimal bacteriocidal concentration (MBC) but for bacteriostatic antibiotics, the MIC may be lower than the MBC. To determine this, an MIC test in broth is first carried out and after incubation, the resultant tubes are subcultured into or onto fresh medium which does not contain antibiotic to determine if viable bacteria are remaining.
Procedure:
- Carry out a broth MIC as above.
- After incubation, subculture each tube onto fresh Mueller Hinton agar and streak out for single colonies. Alternatively, spot inoculate.
- Incubate again at 37oC overnight.
- Growth of the subculture represents viable bacteria remaining in the MIC tubes.