Media
On this page:
MAKING MEDIA - general instructions
MEDIA
MAKING MEDIA
Most media is supplied as dehydrated powders (ready mixed by the manufacturers) which must be mixed with water and sterilized before use. The dry powders are added to the water, boiled to fully dissolve the ingredients (essential if the medium contains agar, which does not dissolve at less than 100oC), dispensed into suitable containers and then autoclaved. Once cooled, bulk media can be stored at 18oC for extended periods. Agar media intended for pouring into Petri dishes is usually autoclaved in bottles containing 100ml or 500ml. One 100ml bottle of agar will normally make 6 plates containing 15-18mls each. The concentration of agar in most solid media is about 1.5% or 15g/l, which results in a firm but not fragile medium with sufficient available water for the growth of bacteria. Broth media is made up in a similar manner, but does not necessarily need to be boiled as it does not contain agar. Solutions are heated until dissolved, dispensed into tubes, bottles or flasks depending on end use, and sterilized in the autoclave. Some reagents added to media are heat sensitive and cannot be autoclaved. These solutions must be added aseptically after autoclaving. These can include solutions of antibiotics and even in some cases, solutions of glucose, which can caramelize in the autoclave. More detail and descriptions can be found in the manuals produced by the major media companies – Oxoid and Becton-Dickinson or BD (BBL/Bacto/Difco). Their websites are below:
For media with CM numbers after the name, refer to the Oxoid catalogue.
BLOOD AGAR
Blood agar is used as a general purpose isolation medium for clinical specimens and for most moderately fastidious bacteria. It consists of an enriched nutrient agar base with 5% added blood, usually either horse or sheep blood. For optimal demonstration of haemolysis by beta haemolytic Streptococci, sheep blood should be used. The medium should be isotonically balanced and with a pH of 7.4 to prevent lysis of the red blood cells. Suitable base media include specialized blood agar bases or Columbia agar.
Blood agar base (CM55)
Lab-Lemco powder 10g
Neutralized peptone 10g
NaCl 5g
Agar 15g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 7.3 +/- 0.2 and bring to the boil on a hotplate. When dissolved, dispense into suitable containers (e.g. 2x500ml bottles or 10x100ml bottles). Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath. Ensure the bottles are cool before adding blood or it will lyse.
Add 5 ml defibrinated horse or sheep blood for each 100 ml of agar base, i.e. 5ml to 100ml or 25 ml to 500ml.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Control Organisms
The following organisms should display typical haemolytic reactions on blood agar plates:
Beta haemolysis (complete clearing) Streptococcus pyogenes
Alpha haemolysis (greening) Streptococcus pneumoniae
Gamma haemolysis (no clearing) Staphylococcus epidermidis
Image copyright American Society for Microbiology
Columbia agar
Special peptone 23g
Starch 1g
NaCl 5g
Agar 10g
Water 1 litre
Make up in the same way as for blood agar base.
Notes on usages:
- Columbia agar plates (without added blood) can be used to test for X and V factor dependence by Haemophilus species.
- Various supplements can be added to make media suitable for the isolation of Campylobacter species and Helicobacter pylori.
- Addition of Fildes extract and egg yolk emulsion produces Nagler plates for the isolation and identification of Clostridium perfringens and blood agar plates made with Columbia base can be used for the reverse CAMP test for the identification of this organism.
- With the addition of serum, Columbia agar can be used to produce Elek plates for the identification of toxin producing strains of Corynebacterium diphtheriae.
BORDET-GENGOU AGAR
This is a highly enriched medium used for the isolation of Bordetella pertussis from clinical specimens. The medium contains potato infusion products and glycerol as well as a high concentration of sheep blood. Originally developed in 1906, the formula has remained relatively unchanged except for a reduction in the amount of blood added (to make it more practicable). The formula includes a higher level of agar than usual to make up for the 15% added blood.
Potato infusion solids 4.5g
NaCl 5.5g
Glycerol 10ml
Agar 20g
Water 1 litre
Add the glycerol to the water. Mix with the dry powders and boil for 1 minute to dissolve. Adjust pH to 7.4+/-0.2. Autoclave at 121oC for 15 minutes. Cool to 50oC and add 15ml defibrinated sheep blood per 85 ml of medium. Pour plates.
Usage
Inoculate plates with nasopharyngeal swabs. Incubate at 37oC in a moist chamber for up to 7 days. Examine after 2 days for the appearance of colonies of B. pertussis (small, domed and glistening). On sheep blood, B. pertussis may be weakly beta haemolytic.
CAMPYLOBACTER MEDIA
There are many media (solid and liquid) designed for the isolation of Campylobacter species. The medium used in practical classes is Skirrow’s medium.
Skirrow’s agar is made from Columbia agar base with the addition of 5-7% laked (or lysed) defibrinated horse blood and a supplement consisting of several antibiotics (vancomycin, trimethoprim and polymyxin B).
Skirrow’s supplement
Vancomycin 5mg
Trimethoprim 2.5mg
Polymyxin B 1250 IU
Reconstitute in 2 ml sterile water and add to 500 ml of melted Columbia agar base held at 50oC. Add laked blood to 5-7% and pour plates.
Quality control
The medium must support the growth of Campylobacter spp. under the following incubation conditions: 42oC in anaerobic jars with Campylobacter specific gaspaks.
CHARCOAL AGAR
Charcoal is known to non-specifically absorb toxic materials, especially toxic fatty acids and its addition to media is essential to enable the growth of certain very fastidious organisms including Bordetella pertussis and Legionella species.
Lab-Lemco powder 10g
Peptone 10g
Starch 10g
Charcoal 4g
NaCl 5g
Nicotinic acid 0.001g
Agar 12g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 7.3 +/- 0.2 and bring to the boil on a hotplate. When dissolved, dispense into suitable containers (e.g. 2x500ml bottles or 10x100ml bottles). Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
5% horse blood can be added for the isolation of Bordetella pertussis.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
CHOCOLATE AGAR
Chocolate agar is used for the growth of fastidious organisms such as Haemophilus species, which cannot derive the necessary nutrients from blood agar. It supplies Haemophilus species with both X and V factors (haem and NAD/NADP respectively) which are required by the organisms for growth. This medium is made from the same ingredients as blood agar and so normal blood agar base or Columbia base can be used. Columbia is superior.
The blood agar base is melted and placed into a 70oC waterbath. When equilibrated, 5% defibrinated horse blood is added and the mixture returned to the waterbath. It is left in the waterbath and periodically mixed until it has turned a chocolate brown colour (approximately 10 minutes). Plates are then poured.
Do not place the blood into the agar until it has cooled to 70oC and do not leave the agar in the waterbath for too long after the addition of blood or the medium will not be uniform (the blood tends to clump).
Control organism
The medium should support the growth of Haemophilus influenzae.
CHROMOGENIC AGAR (UTI)
Chromogenic agars are being used increasingly in diagnostic laboratories to reduce the overall costs associated with the identification of certain bacteria. While these media are more expensive initially than routine agar plates, they can reduce the necessity for further testing and thus reduce costs. The principle of the medium is the detection of a range of enzymes via their effect on various chromogenic substrates, resulting in characteristic coloured colonies.
Urinary tract infections (UTI) are very common and urine specimens make up a large proportion of the specimens received by most diagnostic laboratories. A limited range of bacteria are associated with these infections; the most common cause is E. coli, followed by Staphylococci (coagulase positive and negative), Enterococci, other members of the Enterobacteriaceae such as Proteus mirabilis and Klebsiella pneumoniae and Pseudomonas aeruginosa.
One of the chromogenic substrates (X-gluc) is cleaved by β-glucosidase produced by Enterococci, resulting in blue colonies for this organism. The other chromogenic substrate (red-gal) is cleaved by β-D-galactosidase produced by E. coli, resulting in pink colonies. Other coliforms cleave both substrates resulting in purple colonies. These tests are presumptive and can be confirmed with other biochemical tests such as rapid indole, if necessary. The medium also contains tryptophan, which when cleaved by tryptophan deaminase produced by Proteus spp., results in brown colonies.
The medium is made electrolyte deficient to prevent swarming of Proteus spp. (as for CLED medium below).
Peptone 15g
Chromogenic mix 26.3g
Agar 15g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 6.8 +/- 0.2 . When dissolved, dispense into suitable containers (e.g. 2x500ml bottles or 10x100ml bottles). Autoclave at 121oC for 15 minutes.
Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Note:
The exact nature of the constituents of the chromogenic mix are not released by the company (Oxoid).
Control organisms
E. coli pink colonies
Enterococcus faecalis blue colonies
Proteus mirabilis brown colonies
For more information on chromogenic agars for investigation of urinary tract infections and photos of typical organisms go to the Oxoid website:
http://www.oxoid.com/uk/blue/prod_detail/prod_detail.asp?pr=CM1050&org=&c=uk&lang=en
CYSTINE LACTOSE ELECTROLYTE DEFICIENT (CLED)
CLED is a non-selective differential medium useful for the culture of urine specimens. It supports the growth of both gram positive cocci and gram negative bacilli and hence is less selective than MacConkey agars. As gram positive cocci are significant cause of UTI, and can be inhibited on MacConkey agars, CLED is considered superior for urine testing. Inclusion of lactose and a pH indicator allows the determination of lactose fermentation, which can be used to presumptively identify E. coli, the major cause of UTI. Lack of electrolytes inhibits the swarming of Proteus mirabilis, which form discrete colonies on this medium.
Peptone 4g
Lab-Lemco powder 3g
Tryptone 4g
Lactose 10g
L-cystine 0.128g
Bromothymol blue 0.02g
Agar 15g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 7.3 +/- 0.2 and bring to the boil on a hotplate. When dissolved, dispense into suitable containers (e.g. 2x500ml bottles or 10x100ml bottles). Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Control organisms
E. coli yellow colonies (lactose fermenter)
Proteus mirabilis blue colonies (non-lactose fermenter)
Staphylococci and Enterococci small deep yellow to pale yellow colonies
DNA AGAR
This medium contains polymeric DNA and is used for the detection of DNase enzymes produced by Staphylococci. When exposed to acidic conditions, DNA will precipitate into the agar, producing a cloudy appearance in the plate. If an organism produces a DNase, it will hydrolyse the DNA in the plate to nucleotides, which are not precipitated by acid. Hence DNase positive colonies will have a clear ring around them after development of the plate with HCl, while negative colonies will not have a clear ring around them.
Tryptose 20g
DNA 2g
NaCl 5g
Agar 12g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 7.3 +/- 0.2 and bring to the boil on a hotplate. When dissolved, dispense into suitable containers (e.g. 2x500ml bottles or 10x100ml bottles). Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Usage
Spot inoculate suspect colonies onto the plates and incubate overnight at 37oC. Flood the surface of the plate with 1M HCl and leave to develop for 5 minutes on the bench.
A zone of clear agar remains around colonies of DNase positive organisms while the remainder of the agar becomes cloudy.
Control Organisms
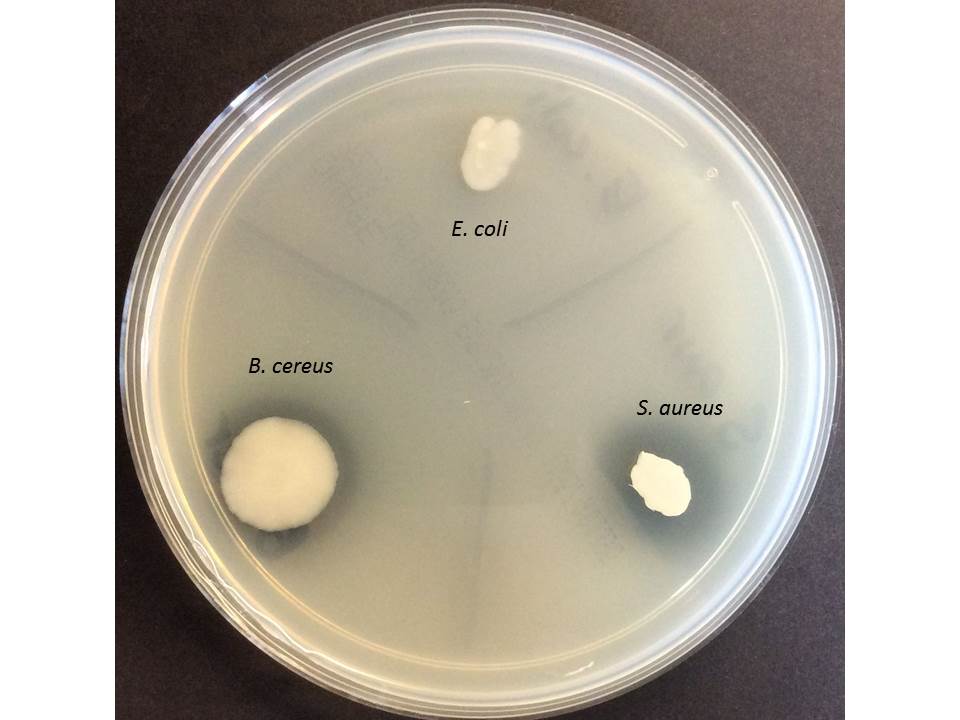
Positive Staphylococcus aureus
Negative Staphylococcus epidermidis
Organisms on image : Positive S. aureus and Bacillus cereus, negative E. coli.
ENTEROCOCCOSEL AGAR
Enterococci species are capable of the hydrolysis of the glycoside, aesculin and can also grow in the presence of bile salts. This differentiates them from the Streptococci which generally cannot grow in bile salts and do not hydrolyse aesculin. The original medium used to test for these characteristics contained 40% bile salts to inhibit other gram positive bacteria but some Enterococci were inhibited at this concentration. Enterococcosel agar was developed with a reduced concentration of bile salts and the addition of sodium azide to inhibit gram negative bacteria, thus making it suitable as an isolation medium for Enterococci from faecal specimens. Enterococci produce black colonies on the medium due to the hydrolysis of aesculin to aesculetin, which reacts with ferric ammonium citrate in the agar to produce a brown/black precipitate around the colonies. Most other bacteria are inhibited.
Note: aesculin is spelt esculin in many sources.
Pancreatic digest of casein 17g
Peptic digest of animal protein 3g
Yeast extract 5g
Oxgall 10g
NaCl 5g
Aesculin 1g
Ferric ammonium citrate 0.5g
Sodium azide 0.25g
Sodium citrate 1g
Agar 13.5g
Water 1 litre
CAUTION: sodium azide is extremely toxic. Consult the technical staff and the safety data sheet before handling this medium.
Suspend dry ingredients in water and boil briefly to dissolve. Adjust pH to 7-1+/- 0.2. Autoclave at 121oC for 15 minutes. Pour plates. Enterococcosel broth can be produced by omitting the agar from the above formulation.
Control Organisms
Enterococcus faecalis produces black colonies after overnight growth;
E. coli and S. pyogenes should be completely inhibited.
EOSIN METHYLENE BLUE AGAR (EMB)
An isolation and differential medium useful for the growth and identification of members of the Enterobacteriaceae, particularly E. coli, which produces colonies with a characteristic metallic sheen on the medium. EMB agar is specified by the American Public Health Association (APHA) protocols for performing coliform counts on water samples.
Peptone 10g
Lactose 10g
K2HPO4 2g
Eosin Y 0.4g
Methylene blue 0.065g
Agar 15g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 6.8 +/- 0.2 and bring to the boil on a hotplate. When dissolved, dispense into suitable containers (e.g. 2x500ml bottles). Autoclave at 121oC for 15 minutes. Cool the medium to 60oC. Before pouring plates, shake the medium to resuspend the precipitate and to oxidize the methylene blue (shake until medium turns blue).
Control Organisms
E. coli : colonies with a dark purple centre and greenish metallic sheen
Enterobacter aerogenes : larger colonies, no sheen, grey-brown centres
GN BROTH
This medium is a mildly selective enrichment medium for enteric gram negative bacteria. It is selective through the activity of various salts and carbohydrates.
Peptone 20g
Glucose 1g
D-mannitol 2g
Tri-sodium citrate 0.5g
K2HPO4 4g
KH2PO4 1.5g
NaCl 5g
Sodium deoxycholate 0.5g
Water 1 litre
Dissolve ingredients in water and adjust pH to 7.0+/-0.2. Dispense in 10ml amounts in 20ml universals. Autoclave 121oC for 15 minutes.
Usage
Small samples of faeces specimens are inoculated to the broth and incubated at 37oC for 18 hours. The broth is then subcultured to selective plates e.g. XLD. Timing is crucial as selection in GN is not absolute, contaminants will eventually outgrow the enteric bacteria.
LACTOSE BROTH
Specified by the APHA for the enumeration of coliforms from water samples and other liquids such as milk. Production of gas (CO2) from the fermentation of lactose is considered presumptive of coliforms. The gas is detected by inclusion of a small fermentation tube (Durham) inverted in the medium. When the tubes of broth are autoclaved, all the air is driven out of the fermentation tube so that no air space or bubbles are seen. When incubated with coliforms in the broth, the organisms produce gas which rises into the small tube and is seen as a bubble.
Lab-Lemco powder 3g
Peptone 5g
Lactose 5g
Water 1 litre
The dry powders are mixed with the water until dissolved. Adjust pH to 6.9+/-0.2. Heating is not necessary. Aliquots are dispensed into large test tubes containing a small inverted Durham tube (see below). Sterilise at 121oC for 15 minutes.
Ensure that there are no air bubbles in Durham tubes before inoculation.
Control Organism
Gas production E. coli or E.aerogenes
LAURYL SULPHATE BROTH
Also recommended by the APHA for the detection of coliforms from water samples and for the enumeration of these organisms by the most probable number (MPN) technique.
The medium contains sodium lauryl sulphate (also known as sodium dodecyl sulphate or SDS) which is a surface active agent (a detergent-like substance). This material acts as selective agent for coliforms in much the same way as bile salts in other media such as MacConkey agars. Gas production from the fermentation of lactose is detected in the same way as for lactose broths. The medium can be made solid by the addition of 1.5% agar.
Tryptose 20g
Lactose 5g
NaCl 5g
K2HPO4 2.75g
KH2PO4 2.75g
Sodium lauryl sulphate (SDS) 0.1g
Water 1 litre
The dry powders are mixed with the water until dissolved. Adjust pH to 6.8+/-0.2. Heating is not necessary. Aliquots are dispensed into large test tubes containing a small inverted Durham tube. Sterilise at 121oC for 15 minutes.
Control organisms
E. coli : gas production at 35oC
Membrane Lauryl Sulphate Broth
Used for the isolation and enumeration of coliforms by the membrane filtration technique.
Peptone 39g
Yeast extract 6g
Lactose 30g
Phenol red 0.2g
Sodium lauryl sulphate 1g
Water 1 litre
Make up as above.
LEGIONELLA ISOLATION MEDIA
Legionella spp. are extremely fastidious and require specialized media for growth and as they are often isolated from environmental samples which may be significantly contaminated, selective agents are necessary. The medium base contains charcoal to absorb toxic fatty acids and is supplemented with yeast extract (buffered charcoal yeast extract or BCYE agar).
BCYE base
Activated charcoal 2g
Yeast extract 10g
Agar 13g
Water 1 litre
Mix and dissolve with heating. Dispense into suitable containers and autoclave at 121oC for 15 minutes. Cool to 50oC.
BCYE Supplement
ACES buffer/KOH 10g/l
Ferric pyrophosphate 0.25g/l
L-cysteine HCl 0.4g/l
α-ketoglutarate 1g/l
Supplied in vials from the manufacturer (Oxoid), each vial sufficient for 100 ml of medium. Reconstitute the vial with 10ml of warm sterile water and aseptically add to 100ml of base agar at 50oC. Final pH should be 6.9 +/- 0.1. Pour plates.
Other selective supplements are available, see the Oxoid manual.
Control organism
The medium should support the growth of Legionella pneumophila.
LURIA-BERTANI BROTH AND AGAR (LB)
This medium is commonly used in biochemistry and molecular biology laboratories for the growth of E. coli strains that are used for cloning purposes. It is more enriched than nutrient broth which contributes to the stability of introduced plasmids in the organisms.
Tryptone 10g
Yeast extract 5g
NaCl 5g
Water 1 litre
Add 15g/l of agar to make LB agar.
Note: some formulations contain 10g/l NaCl. For best results, use Bacto/Difco tryptone and yeast extract if possible.
Dissolve the solids in the water, adjust pH to 7.2+/-0.2 and dispense into suitable containers. Autoclave 121oC for 15 minutes.
MacCONKEY AGARS
There are several different formulations for MacConkey agar, but all contain the same basic ingredients: a nutrient agar base with various formulations of bile salts (as selective agents) and lactose and a suitable pH indicator (as differential agents). They have been developed mainly for the growth and differentiation of gram negative bacilli. Lactose fermenters such as E. coli produce red/pink coloured colonies, non-lactose fermenters such as Salmonella spp. produce yellow or colourless colonies.
The least selective of the MacConkey agars is MacConkey number 1 (Oxoid CM7) which supports the growth of some pathogenic gram positive cocci as well as coliforms. Hence it is useful for urine specimens and wound swabs but it is not sufficiently selective to be suitable for faecal specimens. MacConkey number 1 does not contain additional selective agents other than bile salts.
MacConkey numbers 2 and 3 (CM 109 and 115 respectively) contain different formulations of bile salts and crystal violet as an additional selective agent. They are both suitable for the culture of faecal specimens, but are inhibitory to pathogenic gram positive cocci.
| Number 1 (CM7) | Number 2 (CM109) | Number 3 (CM115) | |
| Peptone | 20g | 20g | 20g |
| Lactose | 10g | 10g | 10g |
| Bile salts | Number 1 5g |
Number 2 1.5g |
Number 3 1.5g |
| NaCl | 5g | 5g | 5g |
| Neutral red | 0.075g | 0.05g | 0.03g |
| Crystal violet | None | 0.001g | 0.001g |
| Agar | 15g | 15g | 15g |
| Water | 1 litre | 1 litre | 1 litre |
| Final pH | 7.4+/-0.02 | 7.2+/-0.02 | 7.1+/-0.02 |
Suspend the ingredients in the water, adjust the pH and boil to dissolve. Dispense into suitable containers and autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Control organisms
Lactose fermenter : E.coli (click on the images below to see a larger view)
Non-lactose fermenter : Salmonella (click on the images below to see a larger view)
MALT EXTRACT BROTH
Useful for the cultivation of fungi, particularly yeasts.
Malt extract 17g
Mycological peptone 3g
Water 1 litre
Dissolve ingredients and adjust pH to 5.4 +/-0.2. Dispense into suitable containers and autoclave at 115oC for 10 minutes. Note lower temperature for autoclaving to prevent caramelization of malt.
Control organisms
The medium should support the growth of Candida albicans but not bacteria e.g. Bacillus cereus.
MANNITOL SALT AGAR
Used as a differential and selective medium for the isolation and identification of Staphylococci. The medium contains high levels of salt which inhibits most organisms except halophiles. Pathogenic Staphylococci are halophilic. Inclusion of mannitol and a pH indicator allows the differentiation of S. aureus (mannitol fermenter) from S. epidermidis (non-fermenter). Inclusion of methicillin at 30mg/l makes the medium selective for methicillin resistant S. aureus (MRSA).
Lab-Lemco powder 1g
Peptone 10g
Mannitol 10g
NaCl 75g
Phenol red 0.025g
Agar 15g
Water 1 litre
Mix the ingredients in a 2 litre flask, adjust the pH to 7.5 +/- 0.2 and bring to the boil on a hotplate. When dissolved, dispense into suitable containers (e.g. 2x500ml bottles or 10x100ml bottles). Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Control organisms
S. aureus : yellow colonies
Coagulase negative Staphylococci : red/pink colonies
Non-halophiles : no growth
METHYL RED/VOGES PROSKAUER MEDIUM (MR/VP)
Many bacteria produce acids as end products from the metabolism of sugars (e.g. lactic acid, acetic acid etc.) while others produce more ethanol and 2,3 butandiol. 2,3 butandiol cannot be assayed directly, but a precursor product, acetoin, can be detected with a simple colour test. Methyl red is a pH sensitive dye which turns red at acid pH and hence will be positive for acid producing organisms but negative for those producing the alcohols. These organisms will be positive for acetoin production, detected by adding Voges Proskauer (VP) reagents to the test. These reagents are an ethanol solution of alpha-naphthol and a strong aqueous solution of KOH. Hence for most organisms, if they are methyl red positive, they will be VP negative and vice versa.
MR/VP medium is basically just a glucose/peptone broth containing potassium phosphate.
Peptone 7g
KH2PO4 5g
Glucose 5g
Water 1 litre
Dissolve the solids in the water, dispense into suitable test-tubes and autoclave at 121oC for 15 minutes.
MR Reagent
Dissolve 0.1g of methyl red dye in 300ml of 95% ethanol. Add 500ml water.
VP-A
40% (w/v) KOH
VP-B
5% (w/v) alpha-naphthol in 100% ethanol
Usage
Bacteria are inoculated into the medium and grown for 18-24 hours at 37oC aerobically.
For the MR test, add 10-15 drops of reagent to the tube. Red colour indicates a positive test.
For the VP test, add 1ml of VP-A to 1 ml of culture, followed by 3ml of VP-B. Positive tests are indicated by the development of a cherry red colouration (may take up to 20 minutes to develop).
Control Organisms
MR positive/VP negative : E. coli
MR negative/VP positive : Enterobacter aerogenes
MILK AGAR
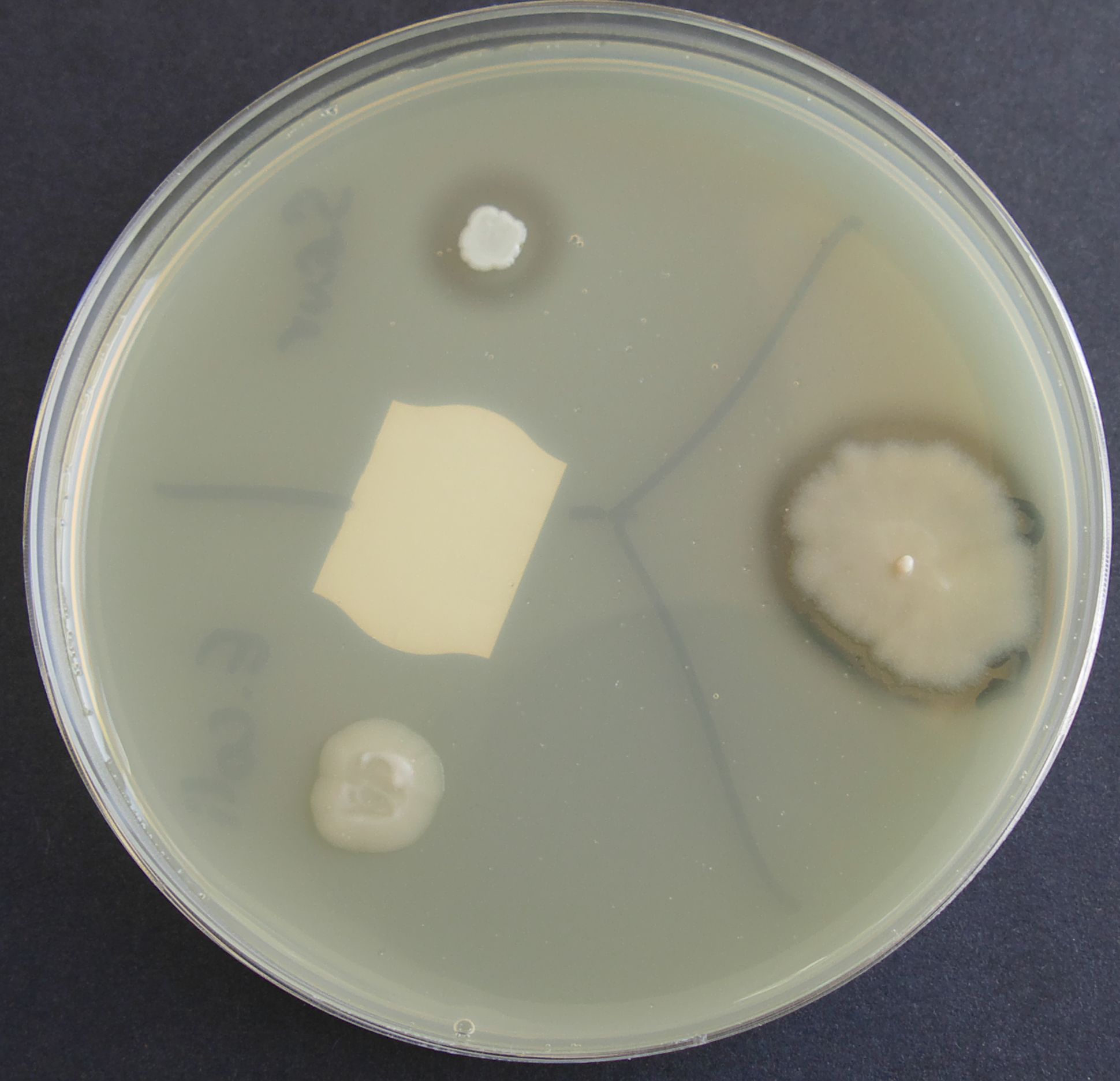
Used for the demonstration of protease production (via the hydrolysis of casein) by Bacillus and Clostridium spp. Proteases degrade casein in the milk solids, resulting in clearing around the protease positive colonies.
Yeast extract 3g
Peptone 5g
Powdered milk 1g
Agar 15g
Water 1 litre
Mix together and boil to dissolve. Adjust pH to 7.2+/-0.2. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
An alternative to adding powdered milk to the formulation is to add fresh, sterile milk to the autoclaved, cooled medium prior to pouring the plates. UHT milk is suitable.
Colonies on milk agar displaying protease activity by clearing of agar around colonies. Positive: Bacillus cereus and E. coli. Negative: S. aureus.
MUELLER-HINTON AGAR AND BROTH
This medium is the recommended medium for antimicrobial sensitivity testing by the protocols developed by the Clinical Laboratory Standards Institute (CLSI) for disc diffusion (Kirby-Bauer) tests. Other media may give erroneous results due to the presence of folate in the medium and other interfering substances. Mueller-Hinton broth is suitable for broth dilution techniques including minimal inhibitory concentration determinations.
Beef, dehydrated infusion from 300g
casein hydrolysate 17.5g
Starch 1.5g
Water 1 litre
(agar) 17g (not in broth)
Agar:
Mix together and boil to dissolve. Adjust pH to 7.4+/-0.2. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Broth:
Dissolve the ingredients in the water. Adjust pH to 7.3+/-0.2. Dispense into suitable containers. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
NUTRIENT AGAR AND BROTH
Nutrient agar and broth are general purpose media used for the cultivation of non-fastidious organisms. They are suitable for the growth of members of the Enterobacteriaceae, Staphylococci and Pseudomonads.
Lab-Lemco powder 1g
Yeast extract 2g
Peptone 5g
NaCl 5g
(agar) 15g (not in broth)
water 1 litre
Agar:
Mix together and boil to dissolve. Adjust pH to 7.4+/-0.2. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
Nutrient agar slopes:
After autoclaving, dispense 10 ml of molten agar into 20ml universal containers and cap tightly. Lay the bottles down on the sloping rack (or at 45o) until solidified. Store at 18oC until required.
Broth:
Dissolve the ingredients in the water. Adjust pH to 7.4+/-0.2. Dispense into suitable containers. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
ONPG BROTH
ONPG or Ortho-nitrophenyl-b-D-galactopyranoside is a substrate for the enzyme beta-galactosidase, which is an enzyme involved in the metabolism of lactose. Breakdown of lactose in bacteria requires 2 enzymes, beta-galactosidase and a permease. True non-lactose fermenters do not produce either of these enzymes, but organisms which lack the permease may appear as late fermenters, even though they produce the beta-galactosidase. ONPG is a synthetic substrate which is cleaved by beta-galactosidase to produce a yellow coloured compound (o-nitrophenol). This occurs even in the absence of the permease. Beta-galactosidase is induced by the presence of lactose, and hence the organism must be derived from a lactose containing medium, reactions from colonies grown on glucose containing media may be delayed as glucose inhibits the enzyme.
Peptone from casein 7.5g
NaH2PO4 0.35g
NaCl 3.75
ONPG 1.5g
Dissolve the ingredients in the water. Adjust pH to 7.4+/-0.2. Dispense into suitable containers. Autoclave at 121oC for 15 minutes.
Usage
Inoculate the tubes with a pure culture of the test bacteria from a MacConkey plate or TSI. Incubate 18-24hours at 37oC. Any degree of yellow colour produced is considered positive. Negative tubes remain colourless/straw coloured.
Control Organisms
Positive : E.coli
Negative : Proteus mirabilis
OXIDATION/FERMENTATION MEDIA (O/F)
There are several different formulations and methods for performing oxidation/fermentation tests; the media and methods chosen depends on the application. Different media formulations are used for gram positive cocci versus gram negative bacilli. The tests basically detect the acid end products of glucose metabolism by the bacteria, with oxygen available and not available. Availability of oxygen is controlled by either inoculating 2 tubes and excluding the oxygen from one with an oil overlay, or by inoculating a deep tube of medium, where oxygen is available on the surface but not at the bottom of the tube. Production of the acid end products is detected by a colour change in a pH indicator incorporated in the medium. The type of indicator may vary for the different formulations.
Hugh and Leifson’s O/F Medium (2 tube method)
Peptone from casein 2.0g
yeast extract 1.0g
sodium chloride 5.0g
di-potassium hydrogen phosphate 0.2g
bromothymol blue 0.08g
agar 2.5g
water 1 litre
also to be added:
glucose 10.0 g/l - filter sterilized.
Dissolve ingredients and autoclave at 121oC for 15 min. Cool to about 50oC, and aseptically add 100 ml/liter of a 10 % filter-sterilized solution of D(+) glucose. The prepared culture medium is dark-green to blue-green in colour and clear and with a pH of 7.1 +/- 0.2 at 25oC. Dispense into tubes to about 5 cm deep.
For the single tube method, more agar should be added to the base medium (to 1.5%) and the tubes should be poured to at least 8cm deep.
The bromothymol blue indicator in the original Hugh Leifson medium is considered inhibitory to Staphylococci and Micrococci and for this reason, bromocresol purple is often used as the pH indicator in O/F tubes for gram positive cocci.
Usage
In the 2 tube method, the organism to be investigated is stabbed into the medium all the way to the bottom of the tube. Two tubes are inoculated and one is overlaid with paraffin oil to a depth of 0.5cm. Both tubes are incubated overnight at 37oC.
| Aerobic tube | Anaerobic tube | Result |
| Yellow | Green | Oxidative |
| Yellow | Yellow | Fermentative |
| Green | Green | Non reactive |
In the single tube method, only one tube is inoculated, again by stabbing all the way to the bottom of the medium. The tube is incubated overnight as above.
| Top of medium | Bottom of medium | Result |
| Yellow | Green | Oxidative |
| Yellow | Yellow | Fermentative |
| Green | Green | Non reactive |
PEPTONE WATER
Peptones are the end products of digestion of proteins by the protease enzyme pepsin. Peptone water is a basal medium to which carbohydrates can be added for fermentation tests (with Andrade indicator), or nitrate added for nitrate reductase tests. It can be used as a recovery or enrichment medium for osmotically injured or otherwise damaged organisms.
Peptone 10g
NaCl 5g
Water 1 litre
Dissolve the ingredients in the water. Adjust pH to 7.2+/-0.2. Dispense into suitable containers. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
Andrade Peptone Water
Andrade indicator
Add 1M NaOH to a 0.5% solution of acid fuchsin until the colour just turns yellow.
Sugar solutions
10% solutions of sugars are added to the base medium to a final concentration of 1%. NOTE: the amount of water added to the base medium must be reduced if adding additional solutions such as sugar solutions. The solutions are added aseptically AFTER autoclaving. Include a Durham tube to demonstrate gas production.
RAPPAPORT-VASSILIADIS BROTH
Used for enrichment of faecal and food/environmental specimens to facilitate isolation of Salmonella spp. Selection is based on the ability of Salmonella to grow at relatively high osmotic pressure and low pH, and to be resistant to malachite green.
Soya peptone 5g
NaCl 8g
KH2PO4 1.6
MgCl2.6H2O 40g
Malachite green 0.04g
Water 1110ml
Dissolve ingredients in water and adjust pH to 5.2+/-0.2. Dispense 10 ml volumes into 20ml universals. Autoclave at 115oC for 15 minutes.
Usage
Place a small quantity of sample material into the broth (alternatively a small aliquot of a pre-enrichment broth e.g. peptone water may be used) and incubate for 18-24 hours at 37oC.
Subculture to suitable differential media e.g. XLD plates after 18 hours.
SABOURAUD DEXTROSE AGAR
This medium is a general purpose agar for the cultivation of yeasts.
Mycological peptone 10g
Glucose 40g
Agar 15g
Water 1 litre
Mix together and boil to dissolve. Adjust pH to 5.6+/-0.2. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
When required, melt the agar in the boiling water bath or microwave. Cool the bottles to 50oC in the waterbath.
Pour into sterile Petri dishes, leave to set on the bench and then dry in the laminar flow hood for 10 minutes with the lids ajar.
Store at 4oC in sealed plastic bags until required.
The medium can be made selective for the culture of clinical specimens likely to contain large numbers of contaminating bacteria. Selective agents include several antibiotics (see Oxoid manual).
SALMONELLA SHIGELLA AGAR (SS)
A selective and differential medium used to isolate Salmonella from faecal specimens. Despite the name, is not suitable generally for the isolation of Shigella spp.
Contains bile salts, thiosulphate, citrate and brilliant green as selective agents. Thiosulphate also acts as an indicator of H2S production, a characteristic of Salmonella spp. H2S positive colonies display a black centre. Inclusion of lactose and a pH indicator allows the differentiation of lactose fermenters from non-lactose fermenters.
This medium has been largely superseded by XLD agar in diagnostic laboratories.
Lab-Lemco powder 5g
Peptone 5g
Lactose 10g
Bile salts 8.5g
Sodium citrate 10g
Sodium thiosulphate 8.5g
Ferric citrate 1g
Brilliant green 0.33mg
Neutral red 0.025g
Agar 15g
Water 1 litre
Ingredients are mixed with water and brought to the boil on a hotplate. Caution must be taken not to inhale dust as the powder is weighed out. After boiling the medium is not autoclaved. Cool to 50oC and pour plates.
Control organisms
The medium should support the growth of Salmonella enterica serotypes and not coliforms such as E. coli. Salmonella will produce clear colonies with black centres.
THAYER-MARTIN AGAR
This medium is used for the selective isolation of Neisseria spp. from clinical specimens. It uses a base of chocolate agar (see above) with the addition of antibiotics, including nystatin to inhibit the growth of yeasts.
THIOSULPHATE CITRATE BILE SALTS SUCROSE AGAR (TCBS)
This medium is a selective medium for the isolation and characterisation of Vibrio cholerae from other gram negative species. It is inhibitory to the Enterobacteriaceae but not to most species of Vibrio. Some strains of Aeromonas hydrophila may grow, producing yellow colonies and may be confused with V. cholerae. Further biochemical testing may be required. TCBS contains a high concentration of salt as a selective agent as Vibrio spp. are frequently marine/halophilic bacteria.
Yeast extract 5g
Bacteriological peptone 10g
Sodium thiosulphate 10g
Sodium citrate 10g
Ox bile 8g
Sucrose 20g
NaCl 10g
Ferric citrate 1g
Bromothymol blue 0.04g
Thymol blue 0.04g
Agar 14g
Water 1 litre
Boil the ingredients to dissolve completely. Adjust pH to 8.6+/-0.02. Do not autoclave. Pour plates immediately. Do not reheat.
Usage
Inoculate faecal or environmental specimens directly to TCBS plates and incubate aerobically at 37oC (for clinical specimens) or 30oC (for environmental specimens).
Vibrio cholerae : yellow colonies/2-3mm diam.
Vibrio parahaemolyticus : blue/green colonies/3-5mm diam
E. coli : no growth
TRIPLE SUGAR IRON AGAR (TSI)
This medium is a complex medium allowing the determination of fermentation of 3 sugars (glucose, lactose and sucrose) and the production of H2S. The sugars are present in different concentrations; lactose and sucrose are present at 10x the concentration of glucose. The agar is poured into slopes, and the suspect colonies are inoculated by stabbing the butt of the agar and streaking the surface of the slope.
Lab-Lemco powder 3g
Yeast extract 3g
Peptone 20g
NaCl 5g
Lactose 10g
Sucrose 10g
Glucose 1g
Ferric citrate 0.3g
Sodium thiosulphate 0.3g
Phenol red q.s.
Agar 12g
Water 1 litre
Mix the ingredients and boil to dissolve. Dispense into large test tubes and autoclave at 121oC for 15 minutes. Remove from autoclave and while still molten lay the tubes on the sloping tray or at 45o until solidified. The butt portion of the slope should be approximately 1cm deep.
Usage
Suspect colonies are streaked down the slope and stabbed into the butt. The tubes are incubated at 37oC overnight. The following reactions may be observed:
| Colour on slope | Colour in butt | H2S? (Blackening) |
Identity |
| Pink | Yellow | No | Non-lactose, non-sucrose fermenter No H2S Possible Shigella |
| Pink | Yellow | Yes | Non-lactose, non-sucrose fermenter H2S producer Possible Salmonella |
| Yellow | Yellow | No | Ferments lactose and/or sucrose No H2S Possible E. coli or Yersinia spp. |
| Yellow | Yellow | Yes | Ferments lactose and/or sucrose H2S producer Possible Proteus spp. |
| Pink | Pink | No | Assacharolytic, possibly Acinetobacter spp. |
If the organism does not ferment either lactose or sucrose, but does ferment glucose, it will produce enough acid in the butt to change the indicator to yellow. On the slope however, there is not enough glucose for this to happen and the alkaline products of peptide degradation will ensure that the slope remains pink. If the organism ferments either lactose or sucrose, enough acid will be produced throughout the medium for it to change to yellow on the slope and in the butt. If the organism does not ferment any of the sugars, no acid is produced and the medium remains pink. H2S production is indicated by a blackening in the butt.
TRYPTONE SOYA BROTH
A general purpose liquid medium, more nutritious than nutrient broth and hence suitable for more fastidious organisms.
Pancreatic digest of casein 17g
Papaic digest of soy bean meal 3g
NaCl 5g
KH2PO4 2.5g
Glucose 2.5g
Water 1 litre
Dissolve the ingredients in the water. Adjust pH to 7.3+/-0.2. Dispense into suitable containers. Autoclave at 121oC for 15 minutes.
Store at 18oC until required.
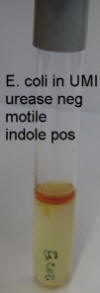
UREA/MOTILITY/INDOLE AGAR (UMI)
This is a composite medium suitable for the determination of motility, production of urease and the detection of indole production. The medium is a semi-solid agar medium through which motile bacteria can move freely. The tube is inoculated by stabbing the medium in a straight line and any growth away from the stab line is indicative of motility. Urease is detected by the incorporation of urea and a pH indicator (phenol red) in the medium. When urea is hydrolysed by urease, ammonia is produced, raising the pH and changing the colour of the medium from pale yellow to bright pink. (Urease can also be detected in a simple urea broth, made by omitting the agar from the formula below.) After incubation, Kovack’s reagent is added to the top of the UMI tube to detect indole, a breakdown product of tryptophan. A positive result is indicative of the presence of a tryptophanase enzyme, and frequently indicates the presence of E. coli.
Peptone 0.95g
Glucose 0.95g
Na2HPO4 1.14g
KH2PO4 0.76g
NaCl 4.75g
Phenol red 0.038g
Tryptone 10.0g
water 950 ml
Agar 2.0g
40% Urea solution 5.0mls
Dissolve all ingredients adding agar last. Do not add 40% Urea. Dispense in 95 ml amounts.
Adjust pH to 6.8+/-0.2.
Autoclave at 115oC for 20 minutes.
Cool to 55oC.
Add 5mls sterile 40%Urea solution to each 95 ml UMI agar base and mix well
Distribute in 3ml amounts into sterile 13x150 tubes using a sterile pipette.
Control organisms
Urease
- Urease Positive : Proteus spp. Pink all through
- Urease Negative : E.coli Buff
Indole – (After addition of Kovacs reagent, added after incubation)
- Positive : E.coli Bright pink at top of tube
- Negative : Proteus spp No colour change
Motility
- Positive : E.coli - growth away from stabline (see above for E. coli or Proteus)
- Negative : Shigella spp. or Yersinia enterocolitica - visible inoculum line, no spreading growth. (see above for Shigella)
VOGES-PROSKAUER MEDIUM
XYLOSE LYSINE DEOXYCHOLATE AGAR (XLD)
XLD is a newer medium for the isolation and presumptive identification of faecal pathogens, particularly Salmonella and Shigella. It is superior to SS agar for the isolation of Shigella. The medium consists of a complex mix of reagents designed to determine xylose, lactose and sucrose fermentation, lysine decarboxylation and H2S production. In this way, it is possible to distinguish between various members of the Enterobacteriaceae.
Yeast extract 3g
L-lysine HCl 5g
Xylose 3.75
Lactose 7.5
Sucrose 7.5
Sodium desoxycholate 1g
NaCl 5g
Sodium thiosulphate 6.8g
Ferric ammonium citrate 0.8g
Phenol red 0.08g
Agar 12.5g
Water 1 litre
Suspend in water and heat gently until boiling. Do not overheat. Cool in a 50oC waterbath. (The medium is not autoclaved.) Pour into plates.
Usage
Most members of the Enterobacteriaceae ferment xylose except Shigella spp. (and Providencia/Edwardsiella) thus Shigella produces red colonies as it does not ferment lactose or sucrose either.
Salmonella produce red colonies with black centres due to H2S production (black) and even though it ferments xylose, the effect of this is counterbalanced by the decarboxylation of lysine, which results in the production of alkaline products turning the colonies from yellow (initially due to xylose fermentation) back to red.
Other lysine decarboxylation positive organisms also tend to ferment either lactose or sucrose (or both) and because these sugars are present in high concentration, acid produced by their fermentation cannot be overwhelmed by the alkaline products of decarboxylation. Thus organisms which are lactose and/or sucrose fermenters tend to produce yellow colonies. An example is Proteus mirabilis (sucrose fermenter) which can produce colonies with a black centre due to H2S production.
E. coli produces yellow colonies on this medium but is frequently inhibited and does not grow well.
YERSINIA SELECTIVE MEDIA
Yersinia enterocolitica has been increasingly recognized as an enteric pathogen but can be difficult to isolate from faecal specimens as it grows slowly at 37oC and can be overwhelmed by competing flora. It requires the use of specialized selective media and an incubation temperature of 25oC. Most of the selective agents are antibiotics.
CEFSULODIN/IRGASAN/NOVOBIOCIN AGAR (CIN)
Agar Base
Special peptone 20g
Yeast extract 2g
Mannitol 20g
Sodium pyruvate 2g
MgSO4 1g
Sodium deoxycholate 0.01g
Neutral red 0.5g
Crystal violet 0.03g
Agar 12.5g
Water 1 litre
Make up in 500ml amounts. Bring ingredients to the boil to dissolve and sterilize at 121oC for 15 minutes. When cool, aseptically add the antibiotic supplement.
Antibiotic supplement
Cefsulodin 7.5mg
Irgasan 2mg
Novobiocin 1.25mg
Dissolve in 2ml of a sterile 50:50 mixture of 100% ethanol and water. Add to 500 ml of base agar as above.
Usage
Plate faecal specimens directly to the medium and incubate initially at 37oC for 18-24 hours, followed by 2-3 days at 25oC.
Yersinia enterocolitica develops small, dark red “bullseye” colonies. Most coliforms do not grow, although some members of the Enterobacteriaceae (Serratia, Citrobacter, Enterobacter) may. These organisms generally produce larger, more mucoid colonies.